
Otto Baumbach in 1928
Alan Gall
Archivist, Institute of Science and Technology, Sheffield

Otto Baumbach in 1928
During the period 2001 - 2004 I had several enjoyable conversations with Geoffrey, son of the German glassblower Otto Baumbach. Otto was a very skilled craftsman, notable for the construction of the delicate apparatus that Ernest Rutherford used in 1908 to confirm the identity of alpha particles as ionized helium, and also for the heated argument with Edward Andrade that seemingly resulted in Otto’s internment for most of the First World War. The following account of Otto’s life is based on a taped interview and correspondence with Geoffrey, supplemented with details from various documents in the possession of the Baumbach family, plus some further research.
Ernest Rutherford had already earned a considerable scientific reputation when, in 1907, he gave up his post at McGill University in Canada to become professor of physics at the University of Manchester. He had been invited to accept the chair by the retiring incumbent, Arthur Schuster, who was able to facilitate the transition by persuading the university authorities to offer Rutherford a generous pay deal. In a letter dated 7th October 1906, Schuster wrote in glowing terms about a new instrument maker and added: ‘We also have a tinsmith next door and a very excellent glassblower within easy reach.’[1] At that time it was not unusual for researchers to construct much of their own equipment. However, Schuster had taken note of the advantages of having at least a few skilled technicians at hand (or rather the disadvantages of being without). His method of accomplishing this at Manchester was to establish agreements with various individuals whereby they would supply the university as a priority when required to do so, but make up their earnings by taking on work for outside customers. This type of cost-effective arrangement provided the services of a tinsmith (William Stelfox), an instrument maker (Charles William Cook) and glassblower Otto Baumbach. These, businesses in their own right, were housed in university property and subject to relocation as the campus developed. Ties with the university were eventually broken and, with the exception of Stelfox, they continued to trade successfully for many years afterwards.
Rutherford had a pretty good idea of the nature of alpha particles when he arrived at Manchester but set out to prove conclusively that they were charged helium. Using the radioactive ‘emanation’ from radium as a source, the alpha particles were allowed to pass through an extremely thin glass tube whilst being prevented from further travel by a second, thicker, glass wall. After allowing the particles time to combine with electrons, spectroscopic analysis confirmed the presence of helium in the space between the two tubes that had been thoroughly evacuated beforehand. A further experiment demonstrated that helium under pressure could not penetrate the thin-walled glass tube.
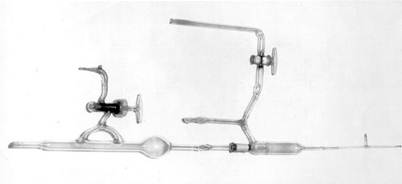
Apparatus for Rutherford’s 1908 alpha particle experiments
© Cavendish Laboratory, Cambridge
In a joint paper with Thomas Royds dated 1909, Rutherford reported:
‘This fine tube_ was sufficiently thin to allow alpha particles from the emanation and its products to escape, but sufficiently strong to withstand atmospheric pressure. After some trials, Mr. Baumbach succeeded in blowing such fine tubes very uniform in thickness.’ [2]
Another scientist of the first rank to be found in Manchester’s
physics department before World War One was Harry Moseley.
H.J.G.Moseley, who was not noted for his praise of others, recorded in 1913:
‘Dear Mr. Baumbach, I have just returned from a holiday and
find here the very beautiful menu holders that you have so
kindly made for me. It was very good indeed of you to take so much
trouble for me and I admire them greatly. With renewed thanks I am
yours sincerely,
Mary Rutherford.’ [4]
The trio of Stelfox, Baumbach and Cook formed a close community, united by their common bond of service to the university. Into the mix came William Alexander Kay, the go-between, laboratory steward in the physics department and trusty assistant to Rutherford. When Charles Cook moved away to Ashby de la Zouch, Otto Baumbach visited him there. Otto also helped Stelfox with financial problems and William Kay kept up his friendships, with Otto at least, for many years after the departure of Rutherford to Cambridge in 1919.
During 1912 the University of Manchester had an extension built at the rear of the physics department. The annex included a room for a glassblower’s workshop and another for the tinsmith. At the official opening in March, Otto demonstrated mercury distillation apparatus made from silica, Bone & Wheeler gas analysis apparatus, an automatic Sprengle pump and Haldane gas analysis apparatus. Charles Cook also provided a display but the tinsmiths, Stelfox, were absent. What part Stelfox actually played in the chain of supply is less well documented than the other two providers. Their Articles of Association stated that they were manufacturers of scientific apparatus and in a taped interview with William Kay, the transcribed record states: ‘And Telefox used to make all the electroscopes, and everything like that, and he [Rutherford] used to think it was a godsend.’ Kay was aged 77 at the time of the interview and, although his memory failed to recall the precise name (or was misheard), he was clearly referring to Stelfox. What is unclear is whether or not he confused Stelfox with Charles Cook who manufactured a range of electroscopes. Stelfox was the least successful of the three. Otto would later tell Geoffrey that the son of the founder was hopeless at business and just let the company fold.
Turning to earlier days, Gottlob Otto Baumbach was born on 10th September 1882 in Niederwilligen, a village surrounded by the pine forests of Thuringia. His father, Frederick, had no known connections with glassblowing and it is believed that he earned a living in Germany by hunting and selling game meat, such as roebuck. Otto studied at the Thüringische Landesfachschule für Glasinstrumententechnik in Ilmenau (Thuringia School for Glass Instrument Technology) then worked briefly for Professor Heike Kamerlingh Onnes (1853-1926) at Leiden University in the Netherlands. Kamerlingh Onnes became the first person to liquefy helium (in 1908), which resulted in the saying that the coldest spot on earth was at Leiden! His laboratory developed into an internationally known centre for studying low temperature physics, as well as a training school for instrument makers and glassblowers.
The date of Otto’s arrival at Manchester has been given variously as 1902[5], and ‘around 1905 after spending a short time in London’[6]. Certainly, it is unlikely to be later than 1904 as a paper presented in May of 1905 gives the credit: ‘We are indebted to the skill of the University glassblower OTTO BAUMBACH for the accurate grinding of these taps, and for the joints by which he succeeded in fusing hard Jena to soft glass.’[7] Initially, Otto was provided with bench space in the chemistry department by Professor Harold Bailey Dixon but after a short time moved to a university building (previously a house) at 10 Lime Grove, one street away. An advertisement of 1911 lists all the firms in the UK supplying the Jena brand of German-made laboratory glassware. Otto’s business was one of four stockists in Manchester (the others being: Baird & Tatlock, Frederick Jackson & Co Ltd and James Woolley, Sons & Co Ltd).[8] It was from Lime Grove that Otto supplied Captain Robert Falcon Scott with sample tubes for the ill-fated Antarctic Expedition of 1910-1912. Why would Scott, based in London, select a supplier in Manchester? It may possibly have been through a recommendation by Arthur Schuster who is known to have advised Scott and others about measurements of the earth’s magnetic field[9].
In 1906 Otto married Hannah Lilian Cowlishaw, the daughter of a commercial traveller. It was his father-in-law, John Charles Cowlishaw, who would help Otto to circumvent the restrictions imposed by the Custodian of Enemy Property on ‘enemy aliens’ trying to resume business activities after the First World War. Hannah and Otto’s first child, Rudolph, died after surviving for only a month, in 1907. Carl (later Charles) Otto Baumbach was born in 1910 and his sister Olga in 1912. The final addition to the family came in 1921 with the birth of John Geoffrey Baumbach, the first names deliberately chosen as typically English.
The argument between Otto and Edward Neville da Costa Andrade has been recounted in a number of Rutherford biographies. Since the accounts are taken from Andrade’s version of events there is more than a possibility of bias. The scenario was that Andrade called in to see Otto only to be greeted with a stream of ultra-patriotic German invective about the superiority of the Kaiser’s army. Andrade, who had studied at Heidelberg and understood the language well, told him to keep his mouth shut or he would find himself in trouble. Geoffrey Baumbach wrote to the New Scientist about this incident, following the publication of an article on Rutherford that had repeated the story.[10]
‘The patriotic outbursts are likely to be quite true. He was only 32 at the time and perhaps somewhat hot headed, as indeed I was at that age. The outbreaks were not spontaneous however. He was not the sort of man to make rude or ill conceived provocative remarks. What has to be remembered however is that part of the strategy of conducting any war is to inflame public opinion against the enemy. My father was an enemy alien surrounded by now with a somewhat hostile population. It is not surprising that he stood his corner now and again, even if a little indiscreetly.’ [11]
World War One turned Otto’s life upside down. Fritz Hartwig, a glassblower who had arrived from Germany to assist Otto before the war, now found himself heir to the business. Perhaps merely by keeping a low profile, Fritz had escaped the unwelcome attentions of the authorities whereas Otto Baumbach and his nephews, the Nauber boys Rudolf and Otto, were incarcerated.[12] Hartwig’s new enterprise, formed in partnership with Alfred Frederick Edwards, and based for a few years at the Baumbach workshop on Bridge Street, was called the Scientific Glassblowing Company.[13]
In anticipation of resuming his profession at the end of the war, and aware that the Scientific Glassblowing Company had replaced him at the university, Otto made an appeal to Rutherford. It was clear that a fresh start had to be made but first he needed to collect whatever debts were outstanding. Rutherford replied:
‘I have received your letter asking whether permission could be granted to you to settle up your business affairs in Manchester and have been making enquiries to see whether anything can be done in the matter. It seems to me desirable that the university matters should be settled before long for as you may have heard I am leaving for Cambridge in July. I have nothing definite to tell you at the moment but will let you know if the prospect improves.’ [14]
A financial settlement, although of a very minor nature, came in 1920 when Chaim Weizmann[15] paid a bill for £4 9s 3d with the comment: ‘I shall be glad to hear from you whether you are continuing your former work.’[16]
Otto now thought it prudent to run his business under a non-German name so began trading as J.C.Cowlishaw, making mainly thermometers, from premises at 403 Chester Road, Old Trafford. Apart from providing the trading name, John Charles Cowlishaw assisted Otto by looking after the books. The business was incorporated on 15th July 1925 and the following year moved to 44 Bridge Street. Meanwhile, the university had been negotiating with both Stelfox Ltd and the Scientific Glassblowing Company because the Electrotechnics department needed extra room.[17] As a result, the glassblowers found premises on nearby Wright Street and Stelfox moved further down Bridge Street, to number 42, next to J.C.Cowlishaw’s works. These two adjacent buildings may have both been owned by Otto as Geoffrey Baumbach spoke of his father showing considerable generosity to Stelfox by providing them with space. However, within three years of moving, the shareholders of Stelfox had voted to put the firm into liquidation.[18]
Rutherford continued to use Otto’s services after taking up the appointment at the Cavendish Laboratory, presumably until the lab appointed its own glassblower in the person of Felix Niedergessas. A letter from Rutherford, written in 1922 survives, although the specification for the requested pump has vanished along with most of the other items from Otto’s early records.
 |
April 8/22 |
|
Dear Baumbach, I shall be glad if you will make within the next fortnight and send to me a pump of the enclosed design – similar to those you made for me in m/c | |
| [Manchester]. |
It is interesting to note that Geoffrey Baumbach had no recollection of his father ever mentioning Felix Nidergesass despite the fact that Otto & Felix ran their own glassblowing businesses in Manchester during the 1920s.[19] In addition, both were German immigrants who had arrived in this country to live in the same district, and of course worked for Ernest Rutherford. Seemingly, James Chadwick was familiar with Felix and his work before going to Cambridge, since it was Chadwick who recommended the glassblower to Rutherford.[20] Felix moved from his home in Manchester to take up his appointment at the Cavendish in approximately 1926.
Sometime in the 1930s, Otto set off with his family to visit Charles Cook. Chas (as he liked to be called) had left the heavily industrialised city of Manchester in about 1922, to live at Ashby de la Zouch where he had bought the Royal Hotel as a rather unusual location for his engineering works, basing the workshops in the disused Spa Baths at the rear of the main hotel building.[21] The young Geoffrey Baumbach remembered the trip because of the anxiety of being late for tea and, on arrival, for the brightly coloured parrots that were kept at the hotel. Another visitor representing the old university days was the engineer William Eccles who wrote to Ludwig Wittgenstein on 28th July 1926, about the trip.[22]
Bernard Lovell arrived at the University of Manchester to join W.L.Bragg’s staff in 1936 and has written of the experience:
‘I tried, but failed absolutely, to interest myself in Bragg’s research and made the bad mistake of trying to restart my thin film work in a place with no facilities and only a poor quality commercial glassblower several streets away’ [23]
This appraisal of Otto is certainly not in keeping with the opinion of others, even the abrasive Edward Andrade.[24] Recently, Sir Bernard Lovell has clarified the situation:
‘As regards Otto Baumbach, he is undoubtedly the glassblower referred to in my article. I fear that my unenthusiastic reference to him is most unfair. I had just come from Bristol where Burrows[25] had succeeded in making a most complex pyrex high vacuum equipment for my research on the deposition of thin films of the alkali metals and this type of apparatus was beyond Baumbach’s experience.’ [26]
Edmund Bowen, in a paper on the Balliol-Trinity Laboratories, Oxford, discussing the problems faced during the 1930s, recalled: ‘There was no workshop, glass-blower, or technical assistance; special glass apparatus was sometimes ordered from Otto Baumbach of Manchester…’[27] Joseph Alexander Gray, in a letter to W.L.Bragg of 19th November 1931, wrote that he was working on the scattering of X-rays at small angles and intended to obtain a special Dewar flask from Baumbach.[28]
At the end of 1929, J.C.Cowlishaw died. Hannah replaced him as a director, until 1931 when she stepped down in favour of Charles Baumbach.[29] Charles became personally involved with some new work for the University of Manchester. When Rutherford resigned as Langworthy Professor at Manchester his successor was William Lawrence Bragg, followed in turn by Patrick Blackett. Blackett arrived in 1937, bringing his team from Birkbeck College: L.Janossy, J.G.Wilson and H.J.J.Braddick. With Bragg at the helm, the research emphasis had been on X-ray crystallography but now this changed to cosmic ray studies. Radiation was now back on the agenda and Charles made many Geiger Counters for Blackett’s team. In particular, Geoffrey Baumbach remembered his brother’s association with Janossy.
The first of the Second World War internment camps on the Isle of Man was set up at Mooragh Promenade, Ramsey after the inhabitants of several boarding houses were served with official notices to vacate on Monday 13th May 1940. It was here that Otto became an internee for the second time.
| A peculiarity of the camp was that it held a number of Finnish prisoners without due regard to their affiliations. Several violent confrontations resulted between pro and anti-Nazi Finns, culmin-ating in the murder of Nestor Huppunen in 1943. By the time of Otto’s release on 19th September 1944, he had spent a total of nine years in captivity during both world wars. According to Geoffrey, the experience did not leave him embittered and he soon resumed his old work. One of his first acts on returning home was to sit down and construct a complex glass manifold. Otto became a naturalised British subject in November 1946. |
 Mooragh internment camp, Isle of Man |
A Compulsory Purchase Order was placed on the Bridgeford Street property (previously named Bridge Street), prompting some drastic action. The sale of ‘Four Gables’, Otto’s house at Mobberley, raised some of the capital for the next venture; relocation to a purpose built factory on a plot of land at Peary Street in Manchester. This was completed in 1963, a year after the death of Otto’s wife Hannah. Otto went to live with his son Geoffrey at Alkrington, near Middleton, Manchester where he died at age 83 in 1966.
Thermometers had been the bread and butter of J.C.Cowlishaw’s activities but a dwindling market for these products demanded a rethink. Then came the lifeline, an opportunity to supply Mather & Platt Ltd with the glass bulbs used in automatic fire-fighting sprinklers. About sixty people were eventually employed hand-making bulbs until it became uneconomical to continue production.
J.C.Cowlishaw moved into electronic circuit assembly work as the requirement for bulbs started to fall. In 1979, after the difficult task of serving redundancy notices on employees of long standing, it became obvious that a voluntary liquidation was the only sensible answer and the company was officially wound-up in 1982.
Per F.Dahl, in Flash of the Cathode Rays, has gone so far as to say that Rutherford’s identification of alpha particles in 1908 is sometimes referred to as the Rutherford-Royds-Baumbach experiment.[30] Others have commented, along with Dahl, that Otto’s internment more or less brought to an end the small number of experiments still possible at Manchester during the Great War.[31] Niels Bohr and Walter Makower, for example, abandoned their investigation when an intricate quartz device constructed by Otto was destroyed by fire.[32] Without doubt, during the period 1907-1914 the glassblowing skill of Otto Baumbach was a timely resource that enabled Rutherford and his co-workers to employ considerably more complicated equipment in their endeavours than might otherwise have been the case. Otto continued after 1919 but with the emphasis on work of a commercial nature, although he was sufficiently well known in the academic world to attract further patronage from university departments. Otto’s second spell of internment came as a bitter blow for a man who had already paid for his youthful indiscretions and given no further offence to his adopted country. Yet despite the deep hurt that this caused, he remained an Anglophile. Friends, family and employees alike remember him for what he was - an intelligent, hardworking man who was modest enough to look back on his life without thinking that he had done anything special.
Acknowledgements
This article would not have been possible without the help of the late Geoffrey Baumbach, and his son Philip. For personal reminiscences I am grateful to Professor Sir Bernard Lovell. Thanks are due to Brian Hosie and Stanley Taylor for discussions about their working life at J.C.Cowlishaw Ltd and to Malcolm Cooper for helpful suggestions with the preparation of this article.’. Details of Internment and the picture of Mooragh Camp came from Yvonne Creswell, Curator of Social History at Manx National Heritage. The photograph of the alpha particle apparatus is courtesy of Keith Papworth at the Cavendish Laboratory, University of Cambridge. Thanks also to Jeff Hughes of the Centre for the History of Science, Technology & Medicine, University of Manchester, under whose guidance much of the research work was undertaken.
Notes and references
[1] Birks, J.B., ed. (1962) Rutherford at Manchester, Heywood & Co, p.52.
[2] Rutherford, E. and Royds, T. (1909) The Nature of the alpha particle from
Radioactive Substances, Philosophical Magazine 17 (6), pp.281-286.
[3] Moseley (1913) The Attainment of High Potentials by the Use of Radium,
Proceedings of the Royal Society of London, Series A 88 (605) p.476.
[4] Ernest Rutherford’s wife writing on 16th August, year not given but
probably prior to 1914 (Source: Baumbach family).
[5] Letter from Thaddeus Trenn to Geoffrey Baumbach 6th
October 1971 (Source: Baumbach family).
[6] In conversation with Geoffrey Baumbach 2001.
[7] Dixon, H.B. and Edgar, E.C. (1906) The Atomic Weight of Chlorine, Philosophical
Transactions of the Royal Society of London, Series A (205) p.176.
[8] Advertisement in Nature, November 16, 1911 p.xxiii
[9] Davies, P.J. (1983) Sir Arthur Schuster 1851-1934, PhD Thesis, UMIST.
[10] Jones, G. The Phantom of the Atom, New Scientist, 28th January 1988.
[11] Undated letter written by Geoffrey Baumbach in early 1988 to the editor of the
New Scientist (Source: Baumbach family).
[12] A beautifully constructed wooden box in the possession of Philip Baumbach is
carved with the words: ‘AUS DER KRIEGSGEFANGENSCHAFT, OLDCASTLE, IRLAND 1916’ (From the prisoner
of war camp, Oldcastle, Ireland 1916).
[13] The Stuart family bought this company when Fritz Hartwig died in 1958 and is still trading today.
[14] Letter from Ernest Rutherford dated 19th May 1919 (Source: Baumbach family).
[15] Weizmann joined the Department of Chemistry at Manchester in 1904. For details see
Reinharz, J. (1985) Chaim Weizmann – The Making of a Zionist Leader, Oxford University Press.
[16] Letter from Weizmann at 16 Addison Crescent, London dated 20th November
1920 (Source: Baumbach family).
[17] Broadbent, T.E. (1998) Electrical Engineering at Manchester University,
The Manchester School of Engineering, University of Manchester, p.110.
[18] By an Extraordinary General Meeting on 13th December 1928.
National Archives records BT31/16779/72909.
[19] Stinson & Niedergesass, Scientific Glassblowers, listed in Slater’s
Directory of Manchester 1921.
[20] Crowther, J.G. (1974) The Cavendish Laboratory 1874-1974, Macmillan.
[21] Hillier, K. (1984) The Book of Ashby-de-la-Zouch, Baron Books, pp. 69-70.
Note that Ken Hillier incorrectly refers to C.W.Cook as an engineer from Nottingham.
[22] Information provided by Prof. Robin Marshall, University of Manchester, from his
correspondence with Monika Seekircher of the Brenner Archives Research Institute, University of Innsbruck.
[23] Lovell, Sir Bernard (1987) Bristol and Manchester – the Years 1931-9, in
Rajkumari Williamson, ed., The Making of Physicists, pp.156-157
[24] Andrade, E.N.da C. (1964) Rutherford and the Nature of the Atom, Anchor Books, p.101.
Heilbron, J.L. (1974) H.G.J.Moseley, The Life and Letters of an English Physicist, 1887-1915,
University of California Press, p.45
[25] J.H. (Johnny) Burrow, glassblower at the University of Bristol
.
[26] Letter from Sir Bernard Lovell to the author, dated 27th March 2003.
[27] Bowen, E.J. (1970) The Balliol-Trinity Laboratories, Oxford 1853-1940, Notes
and records of the Royal Society of London 25 (2), p.235
[28] Royal Institution of Great Britain, ref. W.L.BRAGG/77E/95.
[29] She became a director again in 1938. Companies House, Archive Microfiche.
[30] Dahl, P.F. (1997) Flash of the Cathode Rays, Institute of Physics Publishing, p.332.
[31] The laboratory steward, William Alexander Kay,
was still available to construct apparatus. Although he could also
turn his hand to glassblowing, his skills were not of the same order as Otto’s.
[32] Cockroft, J.D. (1963) Niels Henrik
David Bohr, Biographical Memoirs of Fellows of the Royal Society,
Volume 9, The Royal Society, p.41
Dr. Peter Ford
University of Bath

Last year, 2007, saw events marking the centenary of the death of William Thomson, Lord Kelvin. Articles based on the lectures which took place after our own AGM in Glasgow appear in this issue of our Newsletter. 2007 also marked the centenary of the death of another very famous and influential scientist, which as far as I can tell went completely unnoticed in this country. I am referring to Mendeleev the inventor of the Periodic Table. Charts of the Periodic Table appear in almost every school science laboratory and university chemistry department that I have ever visited. It is probably the most ubiquitous chart in science. I even have in my possession a rather up-market coffee mug on which it is displayed! The purpose of this short article is to summarise his life and to recount how the Periodic Table originated.
Dmitry Ivanovich Mendeleev was born in the Siberian town of Tobolosk on the 8th February 1834. He was the fourteenth and last child of Ivan Mendeleev who was a teacher of Russian Literature. His mother, Maria Kornileva, had a great influence on Mendeleev’s formative years. In 1850, shortly before her death, she took him to St. Petersburg where he was enrolled in the faculty of physics and chemistry at the Main Pedalogical University. He graduated from there in 1855, having obtained an outstanding academic record, and then began to embark on research.
From very early on in his research career Mendeleev was interested in the chemical and physical properties of the elements. He spent the year 1859-60 at the University of Heidelberg working in the research group of Bunsen. This must have been an exciting and influential time for Mendeleev, since Bunsen and his colleague Kirchhoff were actively involved in establishing and developing the principles of spectral analysis, which has become such a powerful investigative technique in science and astronomy. In the course of their work Bunsen and Kirchhoff discovered the elements caesium and rubidium. In 1860 Mendeleev attended the first International Chemical Congress in Karlsruhe. One of the main aims of the congress was to standardise and reach a consensus about some of the basic concepts of chemistry such as atomic, molecular and equivalent weights. The meeting also provided an excellent opportunity for him to meet and discuss with some of the leading chemists of the day.
As the decade of the 1860s progressed, Mendeleev became a rising star in the Russian chemical fraternity. In 1867 he was appointed to the highly prestigious chair of chemistry at the University of St. Petersburg. By this time he had acquired a really detailed knowledge of the existing elements and their compounds.
It was at the start of the nineteenth century that John Dalton, while working in Manchester, had first put forward the idea that each element had a characteristic atomic weight. Mendeleev wrote down the atomic weight and major chemical and physical properties of each element on separate cards. He then placed the cards in vertical columns according to their increasing atomic weight, in a similar manner to playing the card game Patience, which Mendeleev was very fond of doing as a relaxation. Hydrogen, the lightest element, did not seem to fit into any scheme and so he assigned it to a first column all on its’ own. He was perhaps fortunate that helium, the second lightest element, had yet to be discovered, since that too would have given problems within his scheme. From then on things began to make some sense. The next elements in ascending atomic weight are lithium, beryllium, carbon, nitrogen, oxygen and fluorine and Mendeleev placed them in a second vertical column. After fluorine comes sodium and Mendeleev realised that this had similarities to lithium and hence he placed this at the top of a new, third vertical column. Continuing in this manner the next six elements are magnesium, aluminium, silicon, phosphorous, sulphur and chlorine. A pattern was beginning to emerge since, just as lithium and sodium had some similarities at the top of the vertical columns, so did fluorine and chlorine at the bottom. The next element after fluorine in ascending atomic weight is potassium and Mendeleev placed this at the top of a fourth vertical column to be followed by calcium.
It was at this juncture that the first real problem began to appear. The next element in ascending atomic weight known to Mendeleev was titanium and this did not appear to have similarities to either boron or aluminium, which were sitting in the third row of his two previous columns. It is here that Mendeleev’s great insight into the properties of the elements came into play. Around 1869, when he had become seriously involved in this study of the elements, only sixty three of them had been identified while today we know that there are ninety two naturally occurring elements. Hence Mendeleev left a gap in this column, predicting that this was due to an element, which had yet to be discovered. He then placed titanium in the same horizontal row as carbon and silicon where its’ properties seemed to be more in tune. Likewise in his next vertical column Mendeleev predicted that there were again missing elements in the third and fourth rows and, if allowance was made for these, the column would end with bromine with properties which are similar to both fluorine and chlorine. It is difficult to overestimate the intuition that was required by Mendeleev to place gaps in his table, which he believed corresponded to undiscovered elements. Its significance lay in the fact that not only was he able to predict the existence of undiscovered elements, he was also able to suggest their likely atomic weights and some of their properties.
It was some years before Mendeleev’s predictions were verified. In 1875, the French scientist, Professor Wurtz, reported to the French Academy in Paris the discovery in his laboratory of a new element, which had similar properties to aluminium. With understandable Gallic pride the new element was named gallium and had been discovered spectroscopically by Lecoq de Boisbaudran. It was some time before news of this discovery reached Mendeleev in St Petersburg. However, he was then able to point out to the members of the French Academy that the newly discovered element had similar properties to those that he had predicted for the missing element in the fourth vertical column and third row as discussed earlier. In addition he was surprised by the low value obtained by Lecoq de Boisbaudran for the specific gravity of the new element and suggested that he re-determine it using a purer sample. The latter reluctantly agreed to do so and the new value that he obtained was much closer to the value which Mendeleev would have expected. It did much to enhance his prestige in that he seemed to have a much better insight into the properties of the new element than the person who had actually discovered it.
Later in 1879 the Scandinavian chemist Nilson discovered another element, which was called scandium while in 1886 the German chemist Winkler discovered a further element, which not surprisingly was named germanium. These two elements corresponded to the two missing elements predicted by Mendeleev and discussed earlier. By this time Mendeleev had acquired considerable fame and prestige and was feted throughout much of Europe although this did not extend to all of Russia where he had managed to offend the Tsar with some of his liberal political ideas.
Mendeleev had succeeded quite clearly in establishing that there was some underlying pattern or periodicity in the behaviour of the elements as the atomic weight increased. This strongly suggested that there must be some internal structure within atoms, which gives rise to this structure. Here one is entering the domain of the physicist.
In 1897, J.J. Thomson, the Cavendish Professor of Physics at the University of Cambridge, discovered the electron and showed that it had a negative charge and a mass which was some two thousand times smaller than that for the hydrogen atom. Further work quickly established that it was a fundamental constituent of all atoms. Thus hydrogen had one electron, helium, which by 1897 had been discovered, had two, lithium three and so on. Thomson attempted to explain the Periodic Table using his “plum pudding” model of the atom in which the negatively charged electrons moved in concentric rings within a positively charged matrix such that the atom was electrically neutral. Thomson found that after the addition of a certain number of electrons, their mutual repulsion gave rise to instabilities requiring the formation of a new ring. Thus, very qualitatively, he could begin to explain the periodic nature of the atoms.
Thomson’s ideas floundered as a result of some brilliant experiments of Geiger and Marsden carried out in 1909 in the Physical Laboratories of the University of Manchester, which was headed by Rutherford. They bombarded a platinum plate with alpha particles and very occasionally observed large angle scattering of the alpha particles taking place. Rutherford realised that this could only be explained by the atom having a heavy positive nucleus surrounded by planetary electrons analogous to the solar system. Rutherford’s theory of the atom in 1911 was able to account for the observations of Geiger and Marsden. Thomson had also considered a nuclear model for the atom but was forced to reject it on the basis of his sound knowledge of classical physics. Any electron circulating in an orbit around the nucleus would radiate energy and spiral around before crashing into the nucleus. Hence atoms would be unstable in contradiction with the well established fact, going back to the ancient Greeks, of the stability of atoms. Thomson was born in 1856 and was steeply immersed in the ideas of classical physics.
No such inhibitions were felt by the Danish physicist Niels Bohr who was born nearly thirty years after Thomson and was only a teenager at the time that Max Planck first put forward his revolutionary quantum ideas. During his doctoral studies at the University of Copenhagen, Bohr had worked on the electron theory of metals and had found several occasions when classical physics appeared to break down. Bohr believed firmly in the validity of the concept of the nuclear atom. He spent much of 1912 in Rutherford’s laboratory at Manchester and it was here that he began working on his series of seminal papers “On the Constitution of Atoms and Molecules”, which were published in the Philosophical Magazine. Originally the aim of his work was to understand the Periodic Table. However, these papers are best remembered today for containing an explanation of the Balmer series for the spectral lines of the hydrogen atom. This was achieved by using a clever combination of quantum and classical ideas. However Bohr’s work also did much to throw light on the Periodic Table especially the positions in the Table of the rare earth elements. His insight had an important consequence in that colleagues working within his by now famous Institute in Copenhagen were able to isolate without ambiguity a new element. This was called hafnium, which is the Latin name for Copenhagen. Bohr had the considerable coup of being able to announce the discovery of this new element at the end of his Nobel Prize lecture given in Stockholm in 1922.
The Periodic Table that we see today in numerous books and posters is a far cry from the early tentative steps first put forward by Mendeleev beginning around 1867. Nowadays we know of the existence of the noble or rare gases (helium, neon, argon etc.) as well as the artificially created transuranic elements. Mendeleev had to wait until 1955 before element number 101, mendelevium, was named after him. For the rest of his life Mendeleev was constantly trying to refine and develop his ideas. It is right that the Periodic Table is indelibly associated with his name and before 2007 finally disappears over the horizon it is appropriate to mark the centenary of his death and record his enormous contributions in science.
References.
In compiling this article I have made use of the following sources:
Dmitry Ivanovich Mendeleev in Dictionary of Scientific Biography ed. by C.C. Gillispie, Charles Scribner, New York (1970).
The Ascent of Man by J. Bronowski, BBC Publications (1973).
Niels Bohr (1885-1962) articles by P.J. Ford in South African Journal of Science 82, 179-189, (1986): ibid 83, 15-21, (1987); ibid 84, 170-178, (1988).
Seeking Ultimates – An Intuitive guide to Physics by P. T. Landsberg, Institute of Physics Publishing, Bristol and Philadelphia (2000).